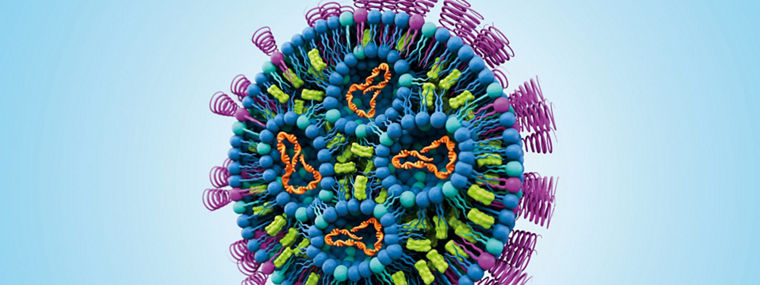
Contract development & manufacturing of custom liposome & lipid nanoparticle products
ANY QUESTIONS LEFT? JUST CONTACT US.
"Our global team of formulation experts is ready to support you with all questions around our product and service portfolio for parenteral drug delivery. Contact us to profit from more than 30 years of application know-how!"
Ahmet Alici
Business Manager