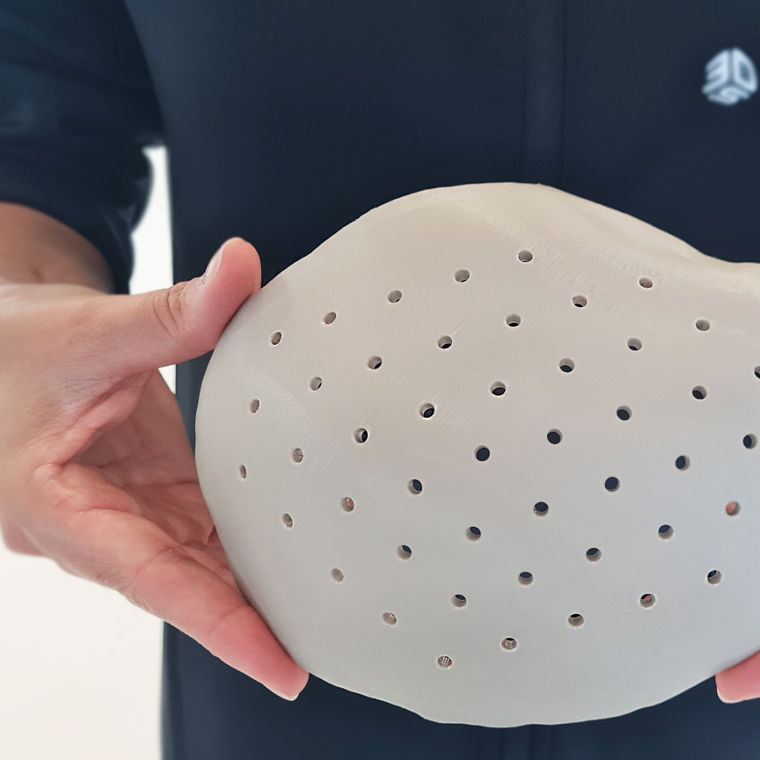
Indeed, the medical devices industry is undergoing significant transformation, driven by a shift towards a continuum-of-care model that emphasizes providing personalized and patient-specific solutions across different settings and stages of life. As the global population ages, there is an increasing demand for innovative technologies tailored to individual patient needs. Advances in areas such as digitalization and robotics are enhancing precision and efficiency in medical devices with increasing complexity, leading to improved patient outcomes and experiences.

Additionally, the pressure to reduce costs is prompting manufacturers to streamline operations and simplify supply chains, while regionalization efforts bolster supply security and responsiveness. At Evonik, we take pride in our global footprint, which allows us to manage our supply chain effectively and support the evolving needs of the medical devices market. We are committed to driving innovations that reflect these trends and enhance the standard of patient-centric care.
Your Innovation Hub for Next-Generation Biomaterials to Create a Life Beyond Limits.
A strong partner for innovating life-changing medical devices. For more than 50 years Evonik has served customers worldwide with innovative materials and services for medical devices. With our global team of experts we support you at every stage of your product development. We offer a comprehensive portfolio of standard and customized biomaterials and value adding services that open up new possibilities for patient-specific treatment, empowering our customers to bring their ideas to life.
Tailored materials and services for medical devices
Evonik provides a wide range of biomaterial-based solutions for the medical devices market. Our next-generation biomaterials are designed to meet the evolving demands of this sector, empowering our customers to swiftly bring impactful medical technologies to market. We offer comprehensive system solutions that include customization, advanced processing platforms, extensive application expertise, and robust regulatory support, all aimed at facilitating the development of cutting-edge medical devices. Our commitment to enhancing patient outcomes underscores the positive impact our materials have on people’s lives, reflecting our dedication to advancing the field of medical technology.
Rooted in science, driven by continuous innovation, and relentlessly committed to the United Nations Sustainable Development Goals, we empower our customers to accelerate time-to-market for new medical technologies that create life beyond limits.
Evonik's portfolio of biomaterials for medical devices
Materials intended for short-term body contact—typically up to 30 days—must meet specific regulatory and performance criteria. Evonik offers a range of materials for applications such as catheters, wound care products, and surgical tools. Our care-grade polymers, including VESTAKEEP®, TROGAMID®, and VESTAMID®, are tailored for short-term use. For wound care and medical aesthetics, epicite® provides advanced healing properties, while ENDEXO® surface modification technology enhances the hemocompatibility of medical devices, reducing thrombosis and biofouling.

| Products | Class of material | Property | Processibiity | Application area |
|---|---|---|---|---|
| Endexo® | Additive technology | Short-term up to long-term body contact | ||
| Epicite® | Biosynthetic cellulose | Short-term body contact | ||
| POLYMER VS | Addition curing silicones | Short-term body contact (Wound care, Explants, etc.) |
| |
| ROHACELL® | Polymethacrylimide (PMI) | Short-term body contact | ||
| TROGAMID® CARE | Transparent polyamides (PA) | Short-term body contact | ||
| VESTAKEEP® Care | Polyether ether ketone polymer ( PEEK) | Short-term body contact |
| |
| VESTAMID® Care ML | Polyamide 12 polymer (PA12) | Short-term body contact | ||
| VESTAMID® Care ME | Polyamide 12 elastomers (PEBA) | Short-term body contact | ||
| VESTENAMER® | Trans-polyoctenamer rubber (TOR) | Short-term body contact | ||
| VESTODUR® | Polybutylene terephthalate (PBT) | Short-term body contact | ||
| VISIOMER® | Specialty Methacrylate Monomers | Short-term body contact Long-term body contact |
|
Devices that remain in the body for more than 30 days require materials with exceptional biostability, mechanical strength, and safety. Evonik’s portfolio includes VESTAKEEP®, DEGACRYL®, and DEGAPLAST®, which perform reliably in long-term implantable applications such as orthopedic devices, stents, and spinal implants. These materials are tested for durability and resistance to physiological stress, ensuring consistent performance over the life of the device.

| Products | Class of material | Property | Processibiity | Application area |
|---|---|---|---|---|
| biocellic® | Biosynthetic cellulose | Long-term body contact | ||
| DEGACRYL® | Polymethyl methacrylate (PMMA) | Long-term body contact | ||
| DEGAPLAST® | Acrylic based resins | Long-term body contact | ||
| NANOCRYL® D | Nanosilica concentrates for acrylic resins | Long-term body contact (Artifical teeth) |
| |
| Polyether ether ketone polymer ( PEEK) | Long-term body contact |
| ||
| VISIOMER® | Specialty Methacrylate Monomers | Short-term body contact Long-term body contact |
|
ANY QUESTIONS? JUST CONTACT US.
Our global team of medical devices experts is ready to support you with all questions around our broad portfolio of products and solutions. Contact us to profit from more than 30 years of application know-how in the field of medical devices and find your individual solution