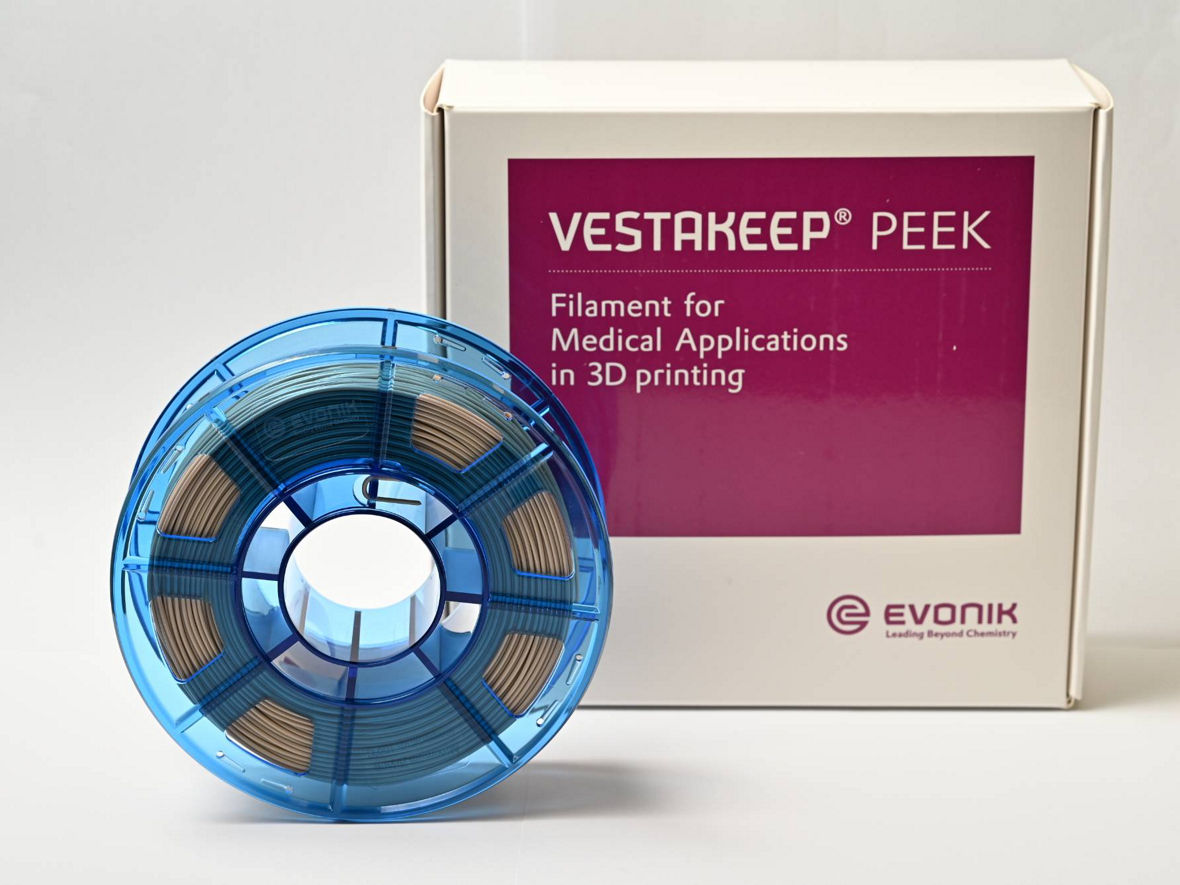
With VESTAKEEP® Care M40 3DF, Evonik offers a polyether ether ketone filament for medical applications with body contact up to 30 days.
The high-performance polymer can be processed in common extrusion-based 3D printing technologies such as fused filament fabrication (FFF) or fused deposition modeling (FDM). With the product launch, Evonik is expanding the possibilities for individual treatment of patients using additive manufacturing.
The new PEEK filament is the latest addition to Evonik’s "Care Grade" line based on the VESTAKEEP® Care M40 high-performance polymer impressing by its
- biocompatibility
- excellent temperature resistance
- excellent chemical resistance
- very good sterilizability, and
- easy handling.
The filament is manufactured under cleanroom conditions and then subjected to strict quality management for medical materials. The range of possible applications extends from patient-specific hearing aids to filigree prostheses and orthoses to surgical drilling aids for dentistry or individual surgical instruments.
ANY QUESTIONS LEFT? JUST CONTACT US.
"Our global team of experts is ready to support you with all questions around VESTAKEEP® PEEK and its applications. Contact us to profit from more than 30 years of application know-how in the field of medical devices and find your individual solution!"
Dr. Philip Engel
Head of Segment Medical Systems Europe